Abstract
Introduction
Patients with diffuse large B-cell lymphoma (DLBCL) with early treatment failure in the rituximab era have poor long-term survival (Maddocks et al, Am J Hematol 2017). However, less is known about patients who experience delayed relapse after failing rituximab-containing induction therapy, and predictors of long-term survival in this population are lacking. We evaluated the course of disease for DLBCL patients treated at Emory University and described the outcomes for those who relapsed at least one year after completing induction therapy.
Methods
Patients ≥ 18 years old with DLBCL evaluated at Emory between January 1, 2000, and January 1, 2017, with documentation of delayed relapse (defined as > 1 year after completion of induction therapy) occurring between these dates were included. We evaluated each patient with regards to induction and salvage treatment regimens, response to treatment, clinical characteristics, and presence of prognostic markers at baseline and time of relapse. Additional demographic and therapeutic variables of interest were also analyzed. The cohort of patients was evaluated with descriptive statistics, and overall survival (OS) and progression-free survival (PFS) were analyzed both from date of original diagnosis and from date of relapse diagnosis. The univariate association of each covariate on OS or PFS was assessed using the COX Proportional hazards model. Survival estimates were calculated the Kaplan-Meier method, and we utilized univariate and multivariable (where feasible) models to assess the association of predictor variables with PFS and OS.
Results
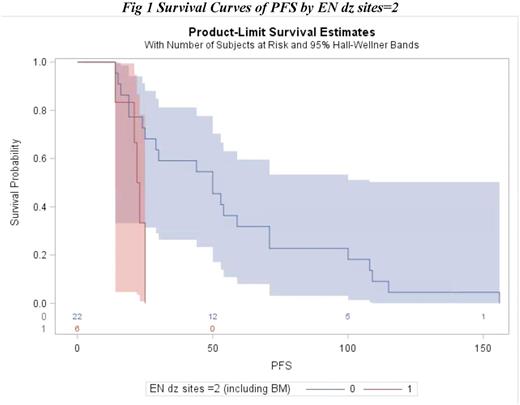
Of the 35 included patients, 60% were male, 71% were white, and 14 patients had stage 1/2 disease and 15 patients had stage 3/4 disease. Thirteen patients had extranodal sites of disease at the time of diagnosis, and 6 patients had at least 2 extranodal sites. The median time from initial treatment to first relapse for the entire cohort was 25 months (range: 13-168 months). Univariate survival analysis of PFS from date of diagnosis demonstrated that there was a significant association between PFS and gender (HR = 0.0183, p = 0.0133), stage (HR = 0.0119, p = 0.0400), and extranodal disease sites at time of diagnosis ≥ 2 (HR = 0.0105, p = 0.0047), with males, patients with limited stage, and patients with < 2 extranodal sites experiencing improved PFS. Additionally, in a multivariable model, extranodal disease sites of < 2 was shown to be a significant predictor for improved PFS., (HR = 0.0112, p = 0.0112). Median PFS from the time of diagnosis for the subgroup with at least 2 extranodal sites at diagnosis was 22 months compared to 50 months for patients < 2 extranodal sites. None of the measured baseline variables predicted OS in the univariate or multivariable analyses.
Conclusions
Among 35 patients with DLBCL experiencing relapse beyond one year after completion of therapy, initial presence of at least 2 extranodal sites was associated with inferior PFS, suggesting that patients presenting with advanced disease remain at risk for relapse even after remaining in remission for a year. These patients should be monitored closely even after achieving complete remission that persists for the first year post treatment. Larger studies are needed to better characterize the predictors of outcomes for patients with DLBCL with later relapse and to identify patients who remain at risk for relapse years after therapy.
Calzada: Seattle Genetics: Research Funding. Flowers: Prime Oncology: Research Funding; National Cancer Institute: Research Funding; Spectrum: Consultancy; Infinity: Research Funding; Gilead: Consultancy; Burroughs Welcome Fund: Research Funding; Seattle Genetics: Consultancy; TG Therapeutics: Research Funding; Janssen Pharmaceutical: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; OptumRx: Consultancy; Acerta: Research Funding; V Foundation: Research Funding; National Institutes Of Health: Research Funding; Research to Practice: Research Funding; Genentech/Roche: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Bayer: Consultancy; Celgene: Consultancy, Research Funding; Millennium/Takeda: Research Funding; Onyx: Research Funding; Eastern Cooperative Oncology Group: Research Funding; Clinical Care Options: Research Funding; Educational Concepts: Research Funding. Cohen: Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; LAM Therapeutics, Inc: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takada: Research Funding; Bioinvent: Consultancy, Membership on an entity's Board of Directors or advisory committees; Infinity: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal